Homepage / AuNP Research Blog / Gold Nanoparticle and Rapid Result Covid19 Tests

ExpressGoldCash - 4.9 star - Customer Reviews
Gold Nanoparticles and
Rapid Result Covid-19 Tests
Originally Posted on 6/18/2020 @ 2:07 pm EDT
Last Edited on 3/26/2025
by Steven Warrenfeltz
Covid-19 Testing (1st Generation)
Most of us have heard, in the news, about the constant rhetoric about the need for faster and more accurate COVID-19 testing.
Two of the 1st generation tests on the market are the PRC (polymerase chain reaction) Diagnostic tests, and the other are Antibody tests.
The PCR diagnostic tests are considered the primary method for detecting if a person has the COVID-19 virus.
Antibody tests are blood tests for those who have conquered the virus to
see if their blood carries any antibodies that can help create a
vaccine for COVID-19.
Both of these tests are reliable; however, both of these 1st-generation tests take hours to get a result, sometimes days, and only medical professionals can administer them.
That's where gold nanoparticles come into the picture; researchers have already incorporated them in creating a better and easier-to-administer 2nd Generation antibody test that doesn't demand that you give up a vile of blood to get a result.
There are some rapid-result diagnostic tests on the market, but it is unknown if they use gold nanoparticles in the testing process because it is proprietary information.
However, several second-generation rapid diagnostic tests are under
development that use gold nanoparticles in their testing process.
The question is, why would gold nanoparticles be used in testing COVID-19? What makes them so special?
Special Properties of
Gold Nanoparticles Used for Testing
COVID-19
Gold nanoparticles are microscopic particles of gold that have been revolutionizing medical research for more than 20 years.
In cancer research, gold nanoparticles have demonstrated several properties that could lead to better cancer treatments with fewer or no side effects. For COVID-19, researchers are incorporating some or all of the following properties of gold nanoparticles for testing the COVID virus.
Bio-Inert
In its physical form, gold is known to be the most non-reactive of all the metals; it does not react with oxygen, which is why it does not tarnish.
Gold nanoparticles are considered bio-inert when introduced into biological tissue. This means they do not trigger or cause an immune reaction and will not contaminate or interfere with the results of a COVID-19 test.
Large Surface Area
Gold nanoparticles have a very large surface area that gives researchers the ability to attach more molecules to them than other types of nanoparticles or biosensors.
The large surface area of gold nanoparticles allows a great number of virus receptors to adhere to the test strip engineered to detect COVID-19.

A Stanford Medicine article published in 2016 stated the following about the incredible surface area of gold nanoparticles:
|
Nanoscale particles also sport tremendous amounts of surface area as compared with larger particles. A cube of gold with sides 1 centimeter long has a total surface area of 6 square centimeters. But the same volume filled with gold nanospheres with diameters of 1 nanometer has a surface area greater than half a football field. - Small wonder: How nanotechnology could detect and treat cancer - Stanford Medicine |
For some, including myself, this fascinating fact about gold nanoparticles is difficult to comprehend.
However, the example from Standford Medicine makes it easier to understand how a small strip of gold nanoparticles can chemically interact and indicate that COVID-19 is present from only a few drops of blood or saliva.
Surface Plasmon Resonance (SPR)
Surface Plasmon Resonance (SPR) happens when focused light hits Gold Nanoparticles, making the electrons in gold nanoparticles resonate and heat up. This form of Surface Plasmon Resonance (SPR) is often used in cancer research to kill cancer cells.

Currently, researchers are utilizing surface plasmon resonance to develop a more precise method for generating rapid results for the COVID-19 diagnosis test.
These tests will likely work by embedding SARS-CoV-2 virus receptors onto a bed of Gold Nanoparticles. SARS-CoV-2 is the virus that causes the COVID-19 disease, in the same regard as the way HIV (virus) causes AIDS (disease).
|
When light is focused through a prism onto the gold nanoparticles, they'll resonate, making the reflected light return at a certain frequency, confirming the presence of COVID-19. This short video offers you a glimpse into how these tests may work. Researchers have stated that diagnostic tests that use this form of Surface Plasmon Resonance will be more accurate, less time-consuming, and less expensive than the current PRC tests that are being used today. |
|
Colloidal Gold &
Localized Surface Plasmon Resonance (LSPR)
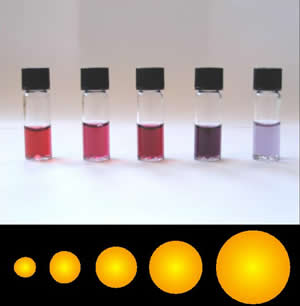
Colloidal gold is a suspension of gold nanoparticles in a fluid, with varying sizes resulting in different colors.
Localized Surface Plasmon Resonance happens when an incandescent light source (the sun, a lamp, etc.) hits gold nanoparticles, causing the electrons in the nanoparticle to resonate.
Resonating gold nanoparticles reflect light at different wavelengths, creating color differences. The color can come from multitudes of nanoparticles suspended in singular form or in comparable-sized clusters.
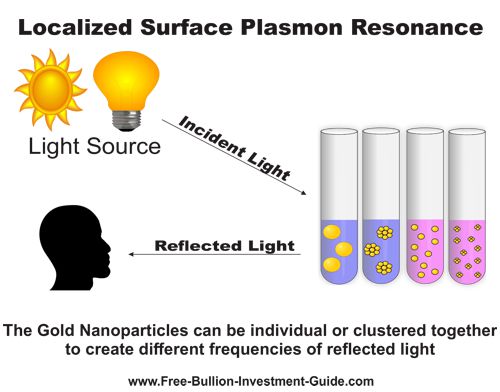
The graphic below offers an additional example of how Localized Surface Plasmon Resonance works.
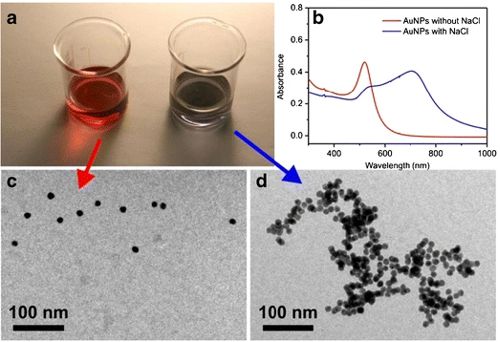
In a 2013 study that tested the reflective properties of gold nanoparticles, researchers added sodium chloride (table salt) to colloidal gold. The sodium chloride made the gold nanoparticles bind together (d), changing the frequency and color of the reflected light (b).
Researchers that use this special property of gold nanoparticles in COVID-19 tests will use receptors of the virus to bind the nanoparticles together. If the virus binds to the SARS-CoV-2 receptor, it will be an indication that the test is positive for COVID-19.
2nd Generation Antibody Tests
The current 2nd generation antibody tests utilize the unique properties of gold nanoparticles, as previously discussed.
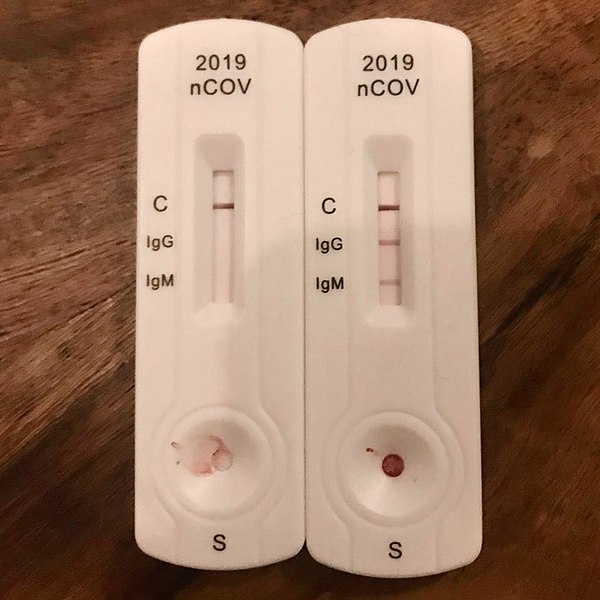
Lateral Flow ImmunoAssay (LFIA) tests are antibody tests that are similar to home pregnancy tests. The devices are composed of an absorbent material strip containing gold nanoparticles.
Attached to the gold nanoparticles are IgG and IgM COVID-19 antibodies; as the blood moves across the absorbent material, molecules in the blood attach to the gold nanoparticles.
If the blood contains antibodies, the gold nanoparticles will collect at each antibody receptor strip on the test, indicating the presence of IgG (immunoglobulin G) and IgM (immunoglobulin M).
|
This short video offers you a glimpse into how these tests may work. Currently, there are several of these COVID-19 gold nanoparticle antibody tests on the market that help detect antibodies in the blood. Below are a few of these test by their manufactures:
|
|
2nd Generation -
Rapid Result Diagnostic Testing Pipeline
There are several research studies in the works to create a new Rapid Result Diagnosis test using gold nanoparticles.
Below is a list of researchers conducting research on a Rapid Result Diagnostic test, along with a brief summary and a link to the corresponding article where the information was obtained.
The pipeline scale graphic at the bottom of each study indicates the researchers' progress in commercializing their concept.
Iceni Diagnostics, a company in the United Kingdom, hopes to have their rapid result diagnosis test out before the next flu season.
The tests will utilize Lateral Flow ImmunoAssay technology, resembling the 2nd Generation Gold nanoparticle antibody tests.
The Iceni Diagnostics's tests will find COVID-19 by using gold nanoparticles coated with "carbohydrate-pathogen recognition to detect the SARS-CoV-2 virus."
Researchers hope the test will hit the market by this fall; they also stated that the test could feasibly defer between the flu vs. coronavirus, which would be, as stated in the article below, "particularly timely."
See the full article about Iceni Diagnostics Rapid Result of COVID-19 tests below.
Iceni Diagnostics Hopes for Home-Use Coronavirus Test this Autumn - Genetic Engineering & Biotechnology NewsUniversity of Texas at Dallas
Next, in treating brain cancer, breaking the blood-brain barrier has been one of the biggest challenges for medical science to overcome.
However, Gold Nanoparticles have shown in research studies that they can help overcome this barrier.
- Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain
- New clinical trial offers hope to patients with glioblastoma
- Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound
Now, cancer researchers at the University of Texas at Dallas who were studying how gold nanoparticles could penetrate deep into the brain have found a way to develop a rapid result COVID-19 test using gold nanoparticles.
The COVID-19 tests would use Surface Plasmon Resonance to get the results from the test in a few minutes.
Below is a small summary from the Science Daily article about how the test works.
|
In the testing method, gold nanoparticles are attached to antibody molecules that can recognize and bind with protein molecules found on the surfaces of viruses. Researchers apply short laser pulses to activate the nanoparticles to generate nanoscale bubbles, or nanobubbles. The accumulation of the nanobubbles signals the presence of
a virus. |
UT Dallas researchers say they're in final stages of creating rapid tests for respiratory viruses like COVID-19 - KHOU-11
McKelvey School of Engineering at Washington University in St. Louis has received Federal Funding to develop a Rapid Result COVID-19 test.
The researchers in this study use a form of Surface Plasmon Resonance to detect SARS-CoV-2, the virus that causes COVID-19.
Their tests, which are called "plasmonic-fluor," use light focused on gold nanoparticle fluorescent biosensors that glow at a very high frequency to indicate that SARS-CoV-2 is present.
At the bottom of the article (below), it states the technology is licensed to Auragent Bioscience and that the company is working on bringing 'plasmonic-flours' COVID-19 testing to the market.
Federal Funding Awarded for Rapid COVID-19 Test - Technology NetworksResearchers at the Institute of Environmental Engineering, ETH Zurich in Switzerland, have developed a COVID-19 Rapid Result test using a form of Surface Plasmon Resonance called "Plasmonic Photothermal Sensing."
This testing platform revolves around using DNA probes that recognize COVID-19 and attaches them to gold nanoparticles.
The tests work by introducing the RNA of SARS-CoV-2 to DNA probes; if the two attach themselves, like a zipper, it will indicate the presence of the virus, whereas if the DNA and RNA do not attach, it would indicate a negative result.
Lasers are used to resonate the gold nanoparticles to make it harder for false positives by shaking loose any imperfect matches.
New test may quickly, accurately detect novel coronavirus: Study - Deccan ChronicleResearchers at the University of Maryland have developed a way to test COVID-19 using Local Surface Plasmon Resonance (LSPR).
In the research, scientists attach COVID-19 gene receptors to gold nanoparticles. When viral RNA is introduced to these gene receptors, the test changes color, confirming the presence of the virus.
If the RNA isn't present in the test, the gold nanoparticle test wouldn't change color.
The University of Maryland lead researcher stated the following about the early results of the study:
|
“Based on our preliminary results, we believe this promising new test may detect RNA material from the virus as early as the first day of infection." - Dipanjan Pan, PhD |
Learn more about the University of Maryland School of Medicine's research from the two links below.
Ming Wang, a professor of civil and environmental engineering at Northeastern University, has converted his proprietary Diabetes monitor into a rapid-result COVID-19 testing device.
This COVID-19 test uses saliva to find its results, which can give an accurate diagnosis in as little as three minutes.
The article, below, states how the testing device works.
|
"The biosensor within the device is designed to use gold nanoparticles to read tiny signals produced as the coronavirus interacts with key protein molecules to hijack human cells and replicate into millions more of itself. The sensor then reads the signals released by those interactions to detect the coronavirus." - Medical Express (May 2020) |
New COVID-19 Treatment?
On the treatment side of COVID-19, recently, the University of Louisville announced a licensing deal with Carlsbad, California-based Qualigen Inc.
The deal involves the college's cancer technology that could treat COVID-19, which uses DNA-coated gold nanoparticles to treat cancers.
The school announced in April that their cancer research also blocked the new coronavirus, SARS-CoV-2, from entering cells and replicating.
The COVID-19 test is still in the early stages; however, the CEO of Qualigen, Michael Poirier, stated the following about the research. “This is a critically important effort that could provide much-needed help to COVID-19 patients worldwide.”

ExpressGoldCash - 4.9 star - Customer Reviews
Other pages, on this Guide, that you
may like...

ExpressGoldCash - 4.9 star - Customer Reviews
Notice:
The charts, commentary, and information on the Free-Bullion-Investment-Guide.com are in no way an endorsement of how you should invest or divest.
|
Support this Guide & Paypal Thank You for Your Support |
|
|
 | |||||

Ad Gloriam Dei
This website is best viewed on a desktop computer.
Keep this Guide Online
& Paypal
Thank You for
Your Support
with Feedly
Search the Guide
| search engine by freefind | advanced |
Premium Canadian Bullion

Give a lasting gift of the iconic Silver Maple Leaf bullion coin [More]
Free Shipping on Orders over $100 (CDN/USA)
or
From the U.K. Royal Mint


Daily
Newsletter
Updated Mintages for
American Gold Buffalo
American Gold Eagle
American Silver Eagle
2024 & 2025
Jerusalem of Gold Bullion
Coin photos
(bottom of page)
Mintages
for
2024
Gold & Silver Mexican Libertad
|
Gold Libertads |
Chinese Gold Coin Group Co.
& Chinese Bullion
Help Us Expand our Audience by forwarding our link
www.free-bullion-investment-guide.com.
Thank You!
Last Month's

In No Particular Order
December 2025
All Articles were Originally Posted on the Homepage